1) Taxonomic inventories, descriptions of new species, and phylogenies
Thanks to a remarkable sampling effort targeted on group of species still under-studied and in under-sampled environments, and thanks to an integrative approach combining morphological and genetic analyses, we have already been able to describe a high number of species new to science (14 new species described so far), and many are still awaiting to be described. Among the new species already described, there are six new species of brown algae in the genus Lobophora (Viera et al. 2020a, 2020b), and eight new species of sponges from several classes (Ruiz et al. 2017, Pérez & Ruiz 2018, Lopes et al. 2018, Fontana et al. 2018, Grenier et al. 2020). Some samples even led to the description of new genera, such as the genus Bidderia in the class of Calcarea sponges (Lopes et al. 2018), described in the French islands of Saint Martin and Guadeloupe (Figure 2B), or the genus Aspiculophora in the class of Homoscleromorpha sponges (Ruiz et al. 2017), described in Martinique, Guadeloupe and Saint Martin.

Figure 2: Examples (underwater photography) of new sponge species discovered in the West Indies and described from material collected during the PACOTILLES campaign. A - Ernstia adunca sp. nov. (Calcarea), Le Rocher du Diamant, Martinique (Fontana et al. 2018) ; B - Bidderia bicolora gen. nov. sp. nov. (Calcarea), Circus-Tintamare, Saint Martin (Lopes et al. 2018), C - Oscarella filipoi sp. nov. (Homoscleromorpha), Anses d'Arlet, Martinique (Pérez & Ruiz 2017) ; D- Oscarella filipoi sp. nov. (Homoscleromorpha) Le Rocher du Diamant, Martinique (Pérez & Ruiz 2017).
Samples of sponges of the Calcarea class collected during PACOTILLES (656 specimens from 10 sites) and analyzed using an integrative taxonomy approach combining morphological and genetic analyses enabled a first inventory to be made. Out of the 485 specimens already analyzed, we have found so far 40 species (8 genera) of Calcinea and 12 species (6 genera) of Calcaronea. Significant variability in Calcarea abundance and species richness at the Lesser Antilles scale is recorded, but these observations remain to be related to the sampling effort deployed on each island. The samples collected in Martinique were specifically the subject of a paper highlighting 11 species from six genera, three of which are new to science (Fontana et al. 2018): Borojevia crystallina sp. nov, Clathrina delicata sp. nov, and Ernstia adunca sp. nov (Figure 2A).
Approximately half of the sponges collected during the PACOTILLES campaign belong to the Demospongiae class, which are by far the most abundant and the best known class of Porifera. The taxonomic study of this class is possible thanks to the coordination of the different expertise available within LIA MARRIO, and it sometimes also involves external collaborators. Today, nearly 75% of these samples have undergone a first treatment, the rest of the collection is currently undergoing a rapid identification at the genus or the Family level, in order to distribute them to our collaborators. In addition to its significant contribution to the inventory of Caribbean sponge fauna, this material also serves the issues of systematics, cosmopolitanism, connectivity on a Greater Caribbean or Western Tropical Atlantic scale and natural product chemistry.
For corals, the number of species recorded during the campaign is 53 (48 Scleractinia and 5 Hydrocorallia). Overal the Caribean region, 116 species of zooxanthelate coral are knowns. The species richness between sites varies from 9 species, for a very degraded lagoon, to 30, for reef areas in good condition. The average richness, estimated at 23 ± 3.4 species per site, is relatively high for the Caribbean region. Siderastrea siderea, Porites astreoides, as well as Hydrocorallaria Millepora alcicornis are species considered ubiquitous since they are present in 97-100% of the stations studied. These are species that are naturally very resistant to environmental alteration and, consequently, their quantitative importance has increased on Caribbean reefs in recent decades as they deteriorate. Conversely, some species that were once common in the Lesser Antilles are now rare. These include, dramatically, the acropore Acropora cerviconis, which occurs in only a few sites. Similarly, in the family Mussidae, species belonging to the genus Mycetophyllia are becoming increasingly rare.
In some taxa, notably sponges of the Homoscleromorpha class, those of the Verongimorpha family (Desmosponges class) and green algae of the Udoteaceae family, the discovery of new species or lineages has overturned the systematics of the group (Ruiz et al. 2017, Lagourgue et al. 2018), calling for taxonomic revisions. However, more generally, the samples collected have allowed the completion of phylogenies through the sequences of taxa for which no genetic data were available prior to this work, and phylogeography for other species for which we have samples from surrounding sites.
2) The Lesser Antilles: a hotspot of marine biodiversity in the North Atlantic?
In brown algae of the genus Lobophora (Fig. 3A-I), our results showed that the Caribbean region stands out as a diversity hotspot in the North Atlantic Ocean. Remarkably, the Eastern Caribbean, in which the Lesser Antilles is located, was found to be the most diverse ecoregion of the Greater Caribbean, with 16 species out of the 19 species recorded in the Atlantic (Fig. 3J). Of these 16 species, 11 are found exclusively in the Greater Caribbean. Biogeographic analyses show that the Greater Caribbean has been a receiving region for Indo-Pacific Lobophora species and a region of diversification, as well as an exporting region for the Northeast Atlantic. The results show that the Greater Caribbean is not an evolutionary dead end for the genus Lobophora, but rather generates and exports diversity.
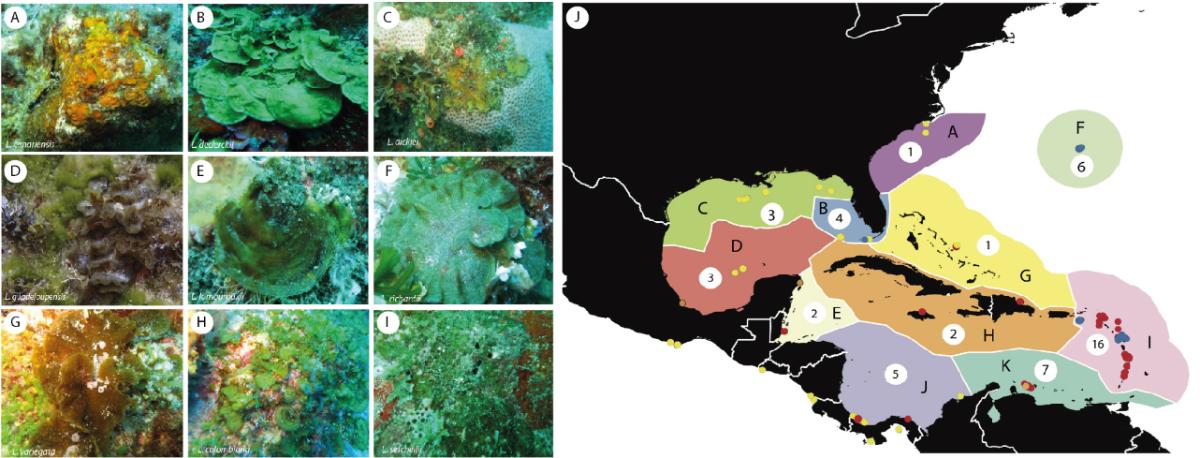
Figure 3: Underwater photographs of some species of Lobophora encountered during the PACOTILLES mission: Lobophora canariensis from Saint Lucia, voucher CP15460 (A); L. declerckii from Saint Lucia, voucher CP15434 (B); L. dickiei sp. nov. from Martinique, voucher CP15086 (C); L. guadeloupensis from Curaçao, voucher CWV0822 (D); L. lamourouxii sp. nov. from Saint Vincent, voucher CP15164 (E); L. richardii sp. nov. from Martinique, voucher CP15127 (F); L. variegata from Saint Martin, voucher CP15730 (G); L. colombiana from Bequia, voucher CP15268 (H); and L. setchellii sp. nov. from Saint Vincent, voucher CP15174 (I). Map representing the diversity of Lobophora species by province (J) with the number of species recorded per province out of the 18 Lobophora species identified in the Caribbean region (Viera et al. 2020a).
Results from samples of green algae of the family Udotecea are remarkably similar (Lagourgue et al. 2018). The results of the delimitation of species from the genetic data revealed, throughout the Caribbean, 18 putative species among which 15 have had a name assigned from morpho-anatomical observations (Figure 4). These 15 taxa belong to the genera Udotea (8 species), Penicillus (4), Rhipocephalus (2), and Rhipidosiphon (1). A species new to science is suspected, as its morpho-anatomical characters do not correspond to any of the known species, but we recommend the prior revision and redefinition of the genus Udotea before it can be described with certainty. Of all the species, 16 were present in the Lesser Antilles, underlining the diversity of this sub-region.
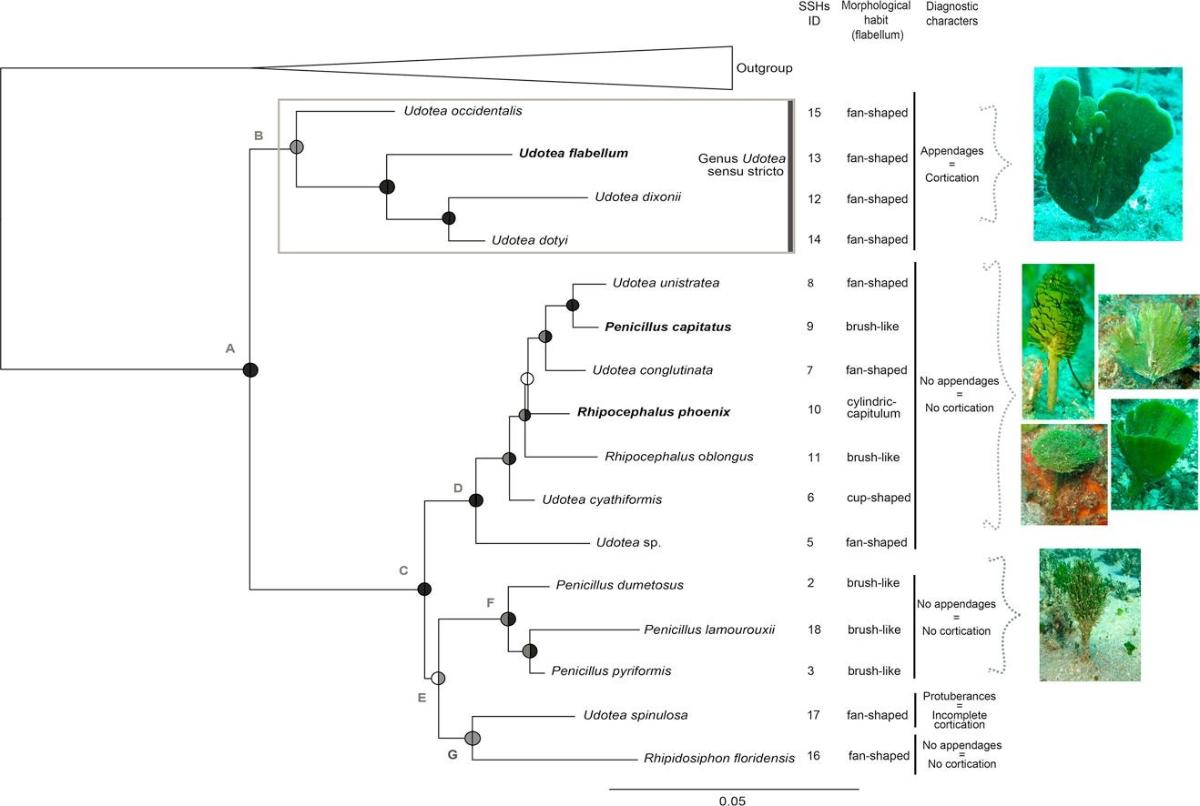
Figure 4: Phylogenetic tree from the sequences of Udoteceae samples collected during the PACOTILLES mission. At each node, the circles represent the ML bootstrap values (left) and the Bayesian posterior probabilities (right): black circles indicate high support (bs > 95; PP > 0.98), dark grey circles represent moderate support (95 > bs > 60; 0.98 > PP > 0.70), and white circles indicate absence of support (bs < 60 and PP < 0.70). The ID of the number of putative species (SSH, according to the species delineation analysis), morphological port and diagnostic traits are shown in the right-hand columns (Lagourgue et al. 2018).
More generally, at the scale of the Lesser Antilles, taxonomic inventories in the groups we studied have shown that despite its peripheral location in the Caribbean region, the Lesser Antilles reveals a very high specific diversity (see the examples previously cited, with the highest number of species recorded for the Caribbean Basin present in the Lesser Antilles). After the work carried out on sponges, La Martinique stands out particularly as a hotspot little equaled on the scale of the Caribbean (Pérez et al. 2017). These results can be partially explained by the inclusion of genetic data in these inventories, which has allowed the identification of several cryptic species. Our study thus underlines the importance of using molecular taxonomic approaches to accurately assess the levels of species diversity and endemism, particularly in peripheral regions that act as evolutionnary incubators. It also underlines that sampling based on molecular identifications can overturn the biogeographic patterns we knew.
3) Genetic diversity and connectivity across the Caribbean Arc
Population samples collected for connectivity analyses showed similar patterns for the three species for which data are already available, although with different levels of genetic differentiation between species. For all three species (the corals Acropora palmata and Porites astreoides, and the sponge Ircinia campana), we observed a significant relationship between genetic and geographic distances between study sites (Figure 5). This pattern of isolation by distance can be explained by larval dispersal limited by geographical distance combined with the specific geographical context of the Lesser Antilles archipelago, i.e. small islands more or less regularly spaced a few kilometers apart and aligned along a north-south axis.
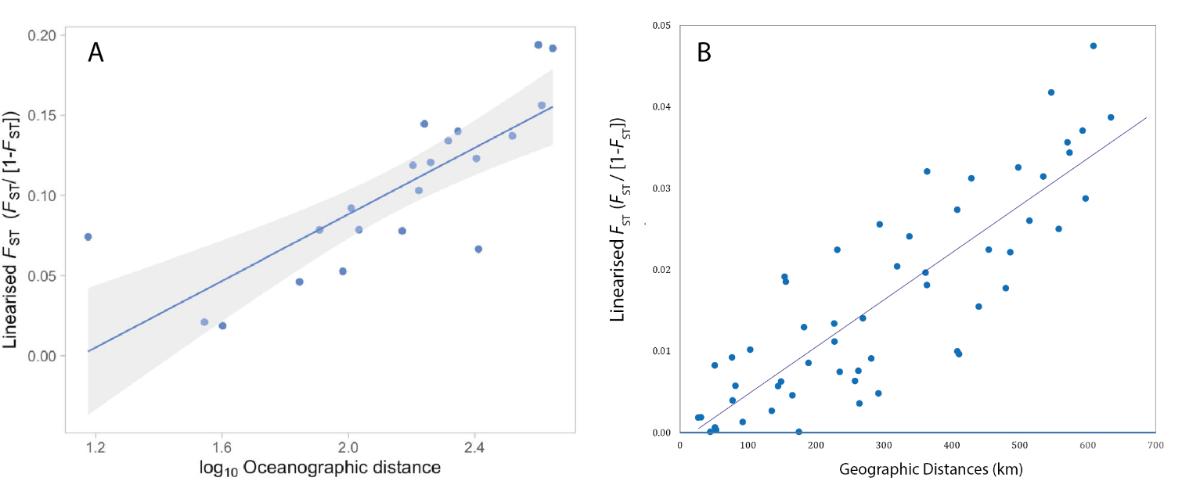
Figure 5: Relationship between genetic distances (standardized Fst) and geographical distances between sites along the Lesser Antilles for Ircina campana (A) and Acropora palmata (B)
However, differences in patterns exist between species, mainly related to the mode of reproduction of the species and the dispersive capacities of the larvae, larval dispersal being the only way for these benthic and fixed species to ensure connectivity between local populations. In Acropora palmata, an endangered species throughout the Caribbean region, analysis of the genetic structure revealed a relatively weak but significant structuring on the scale of the Lesser Antilles, with local populations not genetically differentiated between certain nearby islands (Figure 6). Spatial autocorrelation analyses revealed a larval dispersion limited to less than 1km, with a direction of gene flow mainly oriented towards the north, in accordance with the ocean surface currents in the region. These results suggest that the southernmost populations are potential sources of larvae for the northernmost islands and play a key role in the reseeding of A. palmata populations in the Lesser Antilles (Japaud et al. 2019).
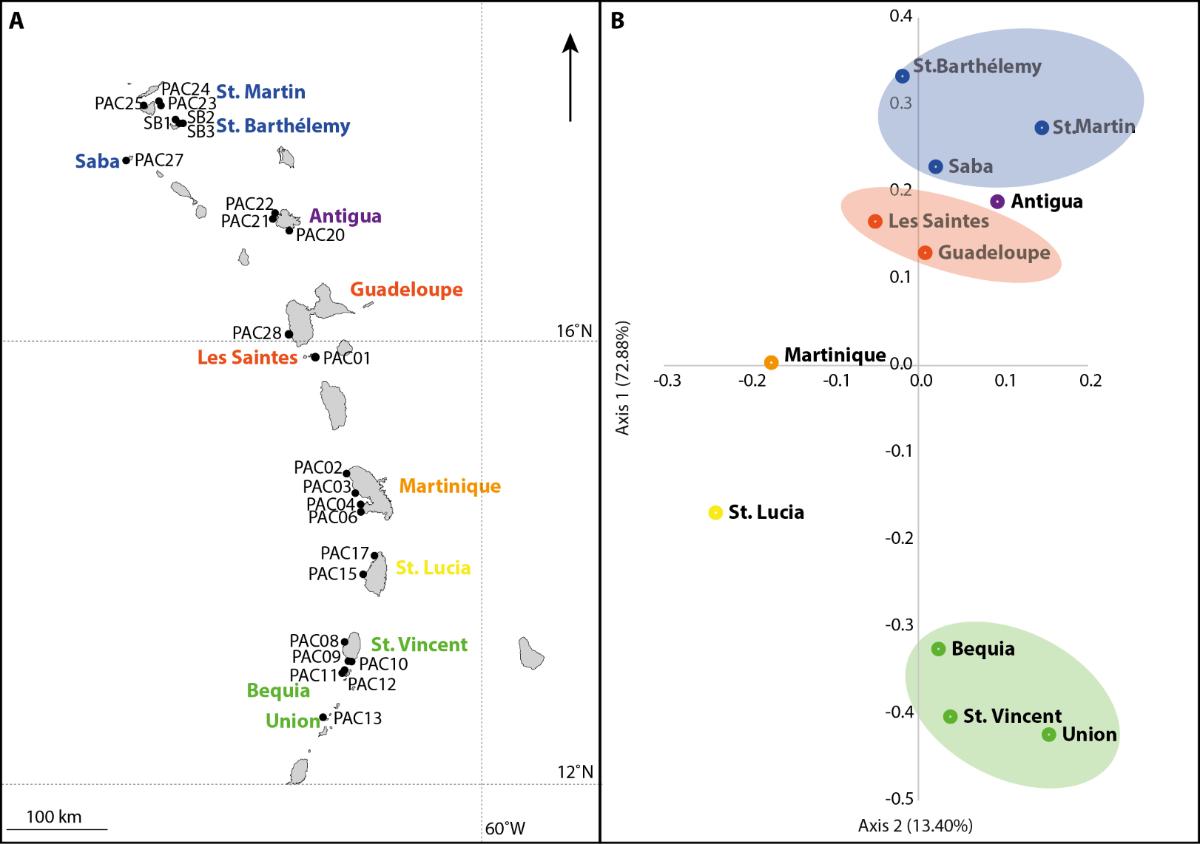
Figure 6: Genetic structure of Acropora palmata coral populations and their geographical distribution in the Lesser Antilles. A: Map of the study area with the sites sampled during the PACOTILLES campaign indicated by a "PAC" label; B: Principal Coordinates Analysis (PCoA) based on genetic similarities between sampled populations using 13 microsatellite loci. Populations sharing the same color (i.e. blue or orange or green) were not found to be genetically differentiated.
On the other hand, for I. campana, a much stronger genetic structuring was observed between the sites sampled in the same study area (Fst ranging from 0.015 to 0.143 between the most distant sites, Guadeloupe and Mayreau) and, in contrast to A. palmata, four genetically differentiated clusters were observed at the Lesser Antilles scale (Figure 7), corresponding to the four sampled islands/areas : (i) Guadeloupe, (ii) Martinique, (iii) St. Lucia, and (iv) St. Vincent and the Grenadines (Griffiths et al. 2020). This strong genetic structuring suggests very limited dispersal, as would be expected in viviparous sponges: although the lifespan of pelagic larvae of I. campana is unknown, it generally varies from a few hours to a few days in sponges. Comparison of these two studies reveals a more limited larval dispersal for I. campana compared to A. palmata. A study being finalized on the oviparous sponge Clathrina aurea could provide additional information (Condor-Lujan, submitted).

Figure 7: Genetic structure of Ircinia campana sponge populations. Each bar represents an individual; the colors represent the membership of a genetic cluster, and the heights of the bars represent the proportions of membership to each cluster. The analysis was first performed on all sites, showing K=4 genetic clusters (a); separate analyses were then performed on the multisite groups identified there (b, c), showing K=4 (b) and K=2 (c) genetic clusters (Griffith et al. 2020a).
Samples of the coral Porites astreoides have also been analyzed using microsatellite markers (Riquet et al. submitted) but are currently being re-analyzed using a RadSeq genomic approach. The first analyses seem to reveal cryptic genetic lines living in sympatry. These genetic analyses will be accompanied by morphometric analyses planned for early 2021.